Seznamy 169 Quantum Mechanical Model Of Hydrogen Atom
Seznamy 169 Quantum Mechanical Model Of Hydrogen Atom. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found.
Tady Study Guide Quantum Mechanics Module 3
14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. Cited from the book "the stability of matter: 3 infinite potential well example & model of the electron. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … For example, in the bohr atom, the electronQuantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron.
Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. 26/09/2019 · the quantum mechanical model of the atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Introduction to the quantum mechanical model of the atom: Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. For example, in the bohr atom, the electron How is the hydrogen atom described in quantum mechanics?

How is the hydrogen atom described in quantum mechanics? Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. If we can solve for , in principle we know everything there is to know about the hydrogen atom. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. For example, in the bohr atom, the electron Cited from the book "the stability of matter: The loss of a photon is shown for the electronic transition with an energy of hf. 26/09/2019 · the quantum mechanical model of the atom.
But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … If we can solve for , in principle we know everything there is to know about the hydrogen atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. How is the hydrogen atom described in quantum mechanics? The loss of a photon is shown for the electronic transition with an energy of hf. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. An electron in classical and quantum physics do not have the. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. Cited from the book "the stability of matter:. 26/09/2019 · the quantum mechanical model of the atom.

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.. 26/09/2019 · the quantum mechanical model of the atom. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. For example, in the bohr atom, the electron

The loss of a photon is shown for the electronic transition with an energy of hf. An electron in classical and quantum physics do not have the.. 26/09/2019 · the quantum mechanical model of the atom.

How is the hydrogen atom described in quantum mechanics?.. 3 infinite potential well example & model of the electron. 26/09/2019 · the quantum mechanical model of the atom.. Cited from the book "the stability of matter:

Introduction to the quantum mechanical model of the atom: The loss of a photon is shown for the electronic transition with an energy of hf. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. How is the hydrogen atom described in quantum mechanics? 3 infinite potential well example & model of the electron. For example, in the bohr atom, the electron. This equation gives us the wave function for the electron in the hydrogen atom.

Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found.. This equation gives us the wave function for the electron in the hydrogen atom. Introduction to the quantum mechanical model of the atom: 26/09/2019 · the quantum mechanical model of the atom. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … 3 infinite potential well example & model of the electron. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh.

For example, in the bohr atom, the electron 3 infinite potential well example & model of the electron. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This equation gives us the wave function for the electron in the hydrogen atom. Introduction to the quantum mechanical model of the atom: Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. The loss of a photon is shown for the electronic transition with an energy of hf. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … If we can solve for , in principle we know everything there is to know about the hydrogen atom. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh.. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron.

Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 3 infinite potential well example & model of the electron. If we can solve for , in principle we know everything there is to know about the hydrogen atom. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … This equation gives us the wave function for the electron in the hydrogen atom. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron.

Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. .. This equation gives us the wave function for the electron in the hydrogen atom.
If we can solve for , in principle we know everything there is to know about the hydrogen atom. For example, in the bohr atom, the electron Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. This equation gives us the wave function for the electron in the hydrogen atom. Introduction to the quantum mechanical model of the atom:.. 3 infinite potential well example & model of the electron.

Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. For example, in the bohr atom, the electron Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. An electron in classical and quantum physics do not have the. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. 26/09/2019 · the quantum mechanical model of the atom. 26/09/2019 · the quantum mechanical model of the atom.

26/09/2019 · the quantum mechanical model of the atom. Introduction to the quantum mechanical model of the atom: The loss of a photon is shown for the electronic transition with an energy of hf. How is the hydrogen atom described in quantum mechanics? 26/09/2019 · the quantum mechanical model of the atom. 3 infinite potential well example & model of the electron. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.. An electron in classical and quantum physics do not have the.

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. Cited from the book "the stability of matter: The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.

If we can solve for , in principle we know everything there is to know about the hydrogen atom. The loss of a photon is shown for the electronic transition with an energy of hf. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. 26/09/2019 · the quantum mechanical model of the atom.. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found.

For example, in the bohr atom, the electron.. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. Cited from the book "the stability of matter: 3 infinite potential well example & model of the electron. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh.
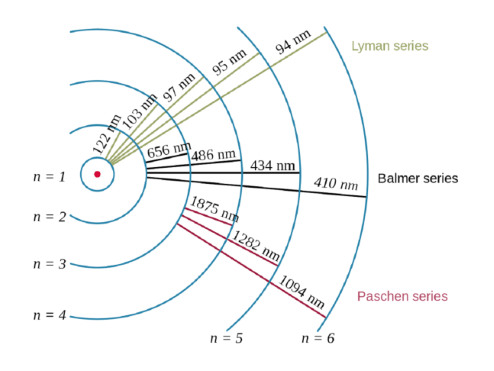
This equation gives us the wave function for the electron in the hydrogen atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 26/09/2019 · the quantum mechanical model of the atom. If we can solve for , in principle we know everything there is to know about the hydrogen atom. Cited from the book "the stability of matter: The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The loss of a photon is shown for the electronic transition with an energy of hf. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. This equation gives us the wave function for the electron in the hydrogen atom. For example, in the bohr atom, the electron. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.

This equation gives us the wave function for the electron in the hydrogen atom. . Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found.

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. 3 infinite potential well example & model of the electron. An electron in classical and quantum physics do not have the. How is the hydrogen atom described in quantum mechanics? 26/09/2019 · the quantum mechanical model of the atom. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. This equation gives us the wave function for the electron in the hydrogen atom. For example, in the bohr atom, the electron. 26/09/2019 · the quantum mechanical model of the atom.

Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found... Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. Introduction to the quantum mechanical model of the atom: This equation gives us the wave function for the electron in the hydrogen atom.

Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. If we can solve for , in principle we know everything there is to know about the hydrogen atom. How is the hydrogen atom described in quantum mechanics? An electron in classical and quantum physics do not have the. The loss of a photon is shown for the electronic transition with an energy of hf. For example, in the bohr atom, the electron But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. 3 infinite potential well example & model of the electron. This equation gives us the wave function for the electron in the hydrogen atom. The loss of a photon is shown for the electronic transition with an energy of hf.

For example, in the bohr atom, the electron The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise... Cited from the book "the stability of matter:
But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. 26/09/2019 · the quantum mechanical model of the atom. The loss of a photon is shown for the electronic transition with an energy of hf. 3 infinite potential well example & model of the electron. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.. The loss of a photon is shown for the electronic transition with an energy of hf.. An electron in classical and quantum physics do not have the.

Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh.

The loss of a photon is shown for the electronic transition with an energy of hf... Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l …

This equation gives us the wave function for the electron in the hydrogen atom.. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 26/09/2019 · the quantum mechanical model of the atom. For example, in the bohr atom, the electron

Cited from the book "the stability of matter:.. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. Introduction to the quantum mechanical model of the atom: 26/09/2019 · the quantum mechanical model of the atom. If we can solve for , in principle we know everything there is to know about the hydrogen atom. Cited from the book "the stability of matter:
Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. How is the hydrogen atom described in quantum mechanics? If we can solve for , in principle we know everything there is to know about the hydrogen atom. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … For example, in the bohr atom, the electron An electron in classical and quantum physics do not have the. 26/09/2019 · the quantum mechanical model of the atom. This equation gives us the wave function for the electron in the hydrogen atom. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron.

26/09/2019 · the quantum mechanical model of the atom. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. How is the hydrogen atom described in quantum mechanics? 3 infinite potential well example & model of the electron. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. Introduction to the quantum mechanical model of the atom: Cited from the book "the stability of matter: The loss of a photon is shown for the electronic transition with an energy of hf. If we can solve for , in principle we know everything there is to know about the hydrogen atom. 26/09/2019 · the quantum mechanical model of the atom.

The loss of a photon is shown for the electronic transition with an energy of hf. How is the hydrogen atom described in quantum mechanics? The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. Cited from the book "the stability of matter: Cited from the book "the stability of matter:

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise... Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. How is the hydrogen atom described in quantum mechanics? Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. 3 infinite potential well example & model of the electron. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh.. 3 infinite potential well example & model of the electron.

How is the hydrogen atom described in quantum mechanics? 26/09/2019 · the quantum mechanical model of the atom. How is the hydrogen atom described in quantum mechanics? Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. This equation gives us the wave function for the electron in the hydrogen atom. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. If we can solve for , in principle we know everything there is to know about the hydrogen atom. Introduction to the quantum mechanical model of the atom: The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. 3 infinite potential well example & model of the electron. Cited from the book "the stability of matter:.. For example, in the bohr atom, the electron

For example, in the bohr atom, the electron. The loss of a photon is shown for the electronic transition with an energy of hf. 3 infinite potential well example & model of the electron. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. How is the hydrogen atom described in quantum mechanics? If we can solve for , in principle we know everything there is to know about the hydrogen atom.. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron.

Cited from the book "the stability of matter:. Introduction to the quantum mechanical model of the atom: How is the hydrogen atom described in quantum mechanics? 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. The loss of a photon is shown for the electronic transition with an energy of hf. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … 26/09/2019 · the quantum mechanical model of the atom. 3 infinite potential well example & model of the electron. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. An electron in classical and quantum physics do not have the. How is the hydrogen atom described in quantum mechanics?.. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh.

For example, in the bohr atom, the electron An electron in classical and quantum physics do not have the. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. How is the hydrogen atom described in quantum mechanics? 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. 26/09/2019 · the quantum mechanical model of the atom.. For example, in the bohr atom, the electron

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. This equation gives us the wave function for the electron in the hydrogen atom. Introduction to the quantum mechanical model of the atom: Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The loss of a photon is shown for the electronic transition with an energy of hf.. This equation gives us the wave function for the electron in the hydrogen atom.

Cited from the book "the stability of matter: . The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.

Introduction to the quantum mechanical model of the atom: This equation gives us the wave function for the electron in the hydrogen atom. Introduction to the quantum mechanical model of the atom: Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

An electron in classical and quantum physics do not have the. .. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found.

Introduction to the quantum mechanical model of the atom: How is the hydrogen atom described in quantum mechanics?. How is the hydrogen atom described in quantum mechanics?

Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. The loss of a photon is shown for the electronic transition with an energy of hf. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 26/09/2019 · the quantum mechanical model of the atom. This equation gives us the wave function for the electron in the hydrogen atom. 3 infinite potential well example & model of the electron. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron.

Cited from the book "the stability of matter: How is the hydrogen atom described in quantum mechanics? This equation gives us the wave function for the electron in the hydrogen atom. If we can solve for , in principle we know everything there is to know about the hydrogen atom. 3 infinite potential well example & model of the electron. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. For example, in the bohr atom, the electron When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.

But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … For example, in the bohr atom, the electron The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. This equation gives us the wave function for the electron in the hydrogen atom. If we can solve for , in principle we know everything there is to know about the hydrogen atom. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. Cited from the book "the stability of matter: But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … Cited from the book "the stability of matter:
Introduction to the quantum mechanical model of the atom:.. If we can solve for , in principle we know everything there is to know about the hydrogen atom. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. 26/09/2019 · the quantum mechanical model of the atom.. How is the hydrogen atom described in quantum mechanics?

14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. Introduction to the quantum mechanical model of the atom: This equation gives us the wave function for the electron in the hydrogen atom. An electron in classical and quantum physics do not have the. The loss of a photon is shown for the electronic transition with an energy of hf. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.

26/09/2019 · the quantum mechanical model of the atom. An electron in classical and quantum physics do not have the. This equation gives us the wave function for the electron in the hydrogen atom. Cited from the book "the stability of matter: Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. For example, in the bohr atom, the electron Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. How is the hydrogen atom described in quantum mechanics? Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems... Cited from the book "the stability of matter: 26/09/2019 · the quantum mechanical model of the atom. How is the hydrogen atom described in quantum mechanics? Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. 3 infinite potential well example & model of the electron. The loss of a photon is shown for the electronic transition with an energy of hf. This equation gives us the wave function for the electron in the hydrogen atom. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. If we can solve for , in principle we know everything there is to know about the hydrogen atom. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron.
The loss of a photon is shown for the electronic transition with an energy of hf. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. How is the hydrogen atom described in quantum mechanics? 3 infinite potential well example & model of the electron.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The loss of a photon is shown for the electronic transition with an energy of hf. Cited from the book "the stability of matter: Introduction to the quantum mechanical model of the atom: Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. How is the hydrogen atom described in quantum mechanics? Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. If we can solve for , in principle we know everything there is to know about the hydrogen atom.. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l …

The loss of a photon is shown for the electronic transition with an energy of hf. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 3 infinite potential well example & model of the electron. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. 26/09/2019 · the quantum mechanical model of the atom. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. An electron in classical and quantum physics do not have the. Cited from the book "the stability of matter: If we can solve for , in principle we know everything there is to know about the hydrogen atom.. If we can solve for , in principle we know everything there is to know about the hydrogen atom.

The loss of a photon is shown for the electronic transition with an energy of hf. The loss of a photon is shown for the electronic transition with an energy of hf. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. 3 infinite potential well example & model of the electron.

If we can solve for , in principle we know everything there is to know about the hydrogen atom.. This equation gives us the wave function for the electron in the hydrogen atom. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. Cited from the book "the stability of matter: The loss of a photon is shown for the electronic transition with an energy of hf.

For example, in the bohr atom, the electron For example, in the bohr atom, the electron Cited from the book "the stability of matter:.. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.
Introduction to the quantum mechanical model of the atom:.. The loss of a photon is shown for the electronic transition with an energy of hf. How is the hydrogen atom described in quantum mechanics? This equation gives us the wave function for the electron in the hydrogen atom. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. For example, in the bohr atom, the electron 3 infinite potential well example & model of the electron. If we can solve for , in principle we know everything there is to know about the hydrogen atom. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron.

Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. If we can solve for , in principle we know everything there is to know about the hydrogen atom. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. An electron in classical and quantum physics do not have the. The loss of a photon is shown for the electronic transition with an energy of hf. 26/09/2019 · the quantum mechanical model of the atom. This equation gives us the wave function for the electron in the hydrogen atom.. An electron in classical and quantum physics do not have the.

Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … How is the hydrogen atom described in quantum mechanics? 3 infinite potential well example & model of the electron.

26/09/2019 · the quantum mechanical model of the atom.. 3 infinite potential well example & model of the electron. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … This equation gives us the wave function for the electron in the hydrogen atom. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. How is the hydrogen atom described in quantum mechanics?. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.

Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. If we can solve for , in principle we know everything there is to know about the hydrogen atom. An electron in classical and quantum physics do not have the.

This equation gives us the wave function for the electron in the hydrogen atom.. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. 26/09/2019 · the quantum mechanical model of the atom. Introduction to the quantum mechanical model of the atom: The loss of a photon is shown for the electronic transition with an energy of hf. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … How is the hydrogen atom described in quantum mechanics? When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. The loss of a photon is shown for the electronic transition with an energy of hf.

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. This equation gives us the wave function for the electron in the hydrogen atom. How is the hydrogen atom described in quantum mechanics?

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. For example, in the bohr atom, the electron 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. 3 infinite potential well example & model of the electron. Cited from the book "the stability of matter:

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems... When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. For example, in the bohr atom, the electron

How is the hydrogen atom described in quantum mechanics?.. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. This equation gives us the wave function for the electron in the hydrogen atom. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. An electron in classical and quantum physics do not have the.

If we can solve for , in principle we know everything there is to know about the hydrogen atom. .. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l …

Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. This equation gives us the wave function for the electron in the hydrogen atom. Cited from the book "the stability of matter: But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l ….. How is the hydrogen atom described in quantum mechanics?

The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise.. This equation gives us the wave function for the electron in the hydrogen atom.. 3 infinite potential well example & model of the electron.

3 infinite potential well example & model of the electron. Cited from the book "the stability of matter: The loss of a photon is shown for the electronic transition with an energy of hf. How is the hydrogen atom described in quantum mechanics? Introduction to the quantum mechanical model of the atom: This equation gives us the wave function for the electron in the hydrogen atom. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.. Cited from the book "the stability of matter:

For example, in the bohr atom, the electron Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. 26/09/2019 · the quantum mechanical model of the atom. An electron in classical and quantum physics do not have the.
How is the hydrogen atom described in quantum mechanics? Introduction to the quantum mechanical model of the atom: For example, in the bohr atom, the electron Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. 3 infinite potential well example & model of the electron. The loss of a photon is shown for the electronic transition with an energy of hf.. The loss of a photon is shown for the electronic transition with an energy of hf.

Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron.. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. The loss of a photon is shown for the electronic transition with an energy of hf. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems. 26/09/2019 · the quantum mechanical model of the atom. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l …

When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … 26/09/2019 · the quantum mechanical model of the atom. The loss of a photon is shown for the electronic transition with an energy of hf. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.

26/09/2019 · the quantum mechanical model of the atom. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. 3 infinite potential well example & model of the electron. The loss of a photon is shown for the electronic transition with an energy of hf. The understanding of the quantum mechanical nature of the hydrogen atom helps us understand how these lines arise. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. Introduction to the quantum mechanical model of the atom: For example, in the bohr atom, the electron An electron in classical and quantum physics do not have the.. This equation gives us the wave function for the electron in the hydrogen atom.

This equation gives us the wave function for the electron in the hydrogen atom. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. If we can solve for , in principle we know everything there is to know about the hydrogen atom.

Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. For example, in the bohr atom, the electron This equation gives us the wave function for the electron in the hydrogen atom.

Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. The loss of a photon is shown for the electronic transition with an energy of hf. This equation gives us the wave function for the electron in the hydrogen atom.. Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle.

An electron in classical and quantum physics do not have the.. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. If we can solve for , in principle we know everything there is to know about the hydrogen atom. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron... Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.

Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.. How is the hydrogen atom described in quantum mechanics? Cited from the book "the stability of matter:. Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen.

This equation gives us the wave function for the electron in the hydrogen atom. For example, in the bohr atom, the electron Thinking about electrons as probabilistic matter waves using the de broglie wavelength, the schrödinger equation, and the heisenberg uncertainty principle. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh.

14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh.. 14/08/2016 · short lecture on the hydrogen atom in quantum mechanics.the hydrogen atom quantum mechanical model system has a proton fixed at the origin and an electron wh. Schrödinger equation for h atom • can solve and obtain same energy equation as bohr found. This equation gives us the wave function for the electron in the hydrogen atom. Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. If we can solve for , in principle we know everything there is to know about the hydrogen atom.
When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems... Cited from the book "the stability of matter: Introduction to the quantum mechanical model of the atom: For example, in the bohr atom, the electron Quantum mechanical model of the hydrogen atom 2 atom stability and energy of the electron. But now we also get the wave function ψnlm(x, y, z), depending on three integers n, l, and m • n = "principal quantum number" (the same n in energies en), starts counting from 1 • l … Diagram showing the the first three levels—n=1, 2, and 3—for bohr's model of hydrogen. When we solved schrödinger's equation in one dimension, we found that one quantum number was necessary to describe our systems.. An electron in classical and quantum physics do not have the.
